Qualitative Analysis of Organic Compounds
Qualitative Analysis of Organic Compounds: Overview
This Topic covers sub-topics such as Qualitative Analysis of Organic Compounds, Lassaigne's Test, Identification of Functional Groups, Detection of Carbon and Hydrogen, Detection of Nitrogen, Detection of Phosphorus and, Detection of Sulphur
Important Questions on Qualitative Analysis of Organic Compounds
The compound formed in the positive test for nitrogen with the Lassaigne's solution of an organic compound is
An organic compound having molecular mass is found to contain and while, rest is oxygen. On heating it gives along with a solid residue. The solid residue gives violet colour with alkaline copper sulphate solution. The compound is:
In any ferric salt, on adding potassium ferrocyanide a Prussian blue colour is obtained, which is mainly due to the formation of
In Lassaigne's extract of organic compound both nitrogen and sulphur are present, which gives blood-red colour with due to the formation of
Match List-I with List-II.
|
List-I Element detected |
List-II Reagent used/Product formed |
||
| Nitrogen | |||
| Sulphur | |||
| Phosphorus | |||
| Halogen | |||
Choose the correct answer from the options given below:
What happens when is heated
Balance the above reaction and find the missing reactant in the given reaction?
How many compound(s) gives blood red colour with in Lassaigne's test?
(a)
(b)
(c)
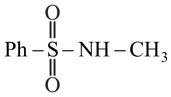
(d)
(e)
(f)
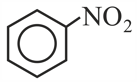
Prussian blue is obtained by mixing together aqueous solution of salt with
There are four test tubes containing dilute and solutions. Which of the following reagents will help in the identification of ?
Number of compounds which can show unsaturation test with
(a)

(b)

(c)
(d) Styrene
(e) Cinnamic acid
(f)
(g)
(h)
(i)
Compound gives negative tests with the following reagents.
(a)
(b) -Dinitrophenylhydrazine
(c) metal.
It gives two monochloro structural isomers.
Identify ''.
Describe the reactions involved in the detection of nitrogen in an organic compound by Lassaigne method.
Explain the method for the detection of phosphorus.
What is the colour of the ammonium phosphomolybdate precipitate which is formed during the detection of phosphorus?
For the detection of phosphorus, the organic compound after fusion with is extracted with water, boiled with and then ammonium molybdate added to it. A yellow precipitate is obtained which is due to formation of:
Discuss in brief the principle of estimation of phosphorus.
How carbon and hydrogen are detected in an organic compounds?
What happens wwhen an organic compound is heated with ?
The colour of ammonium phosphomolybdate is :
